
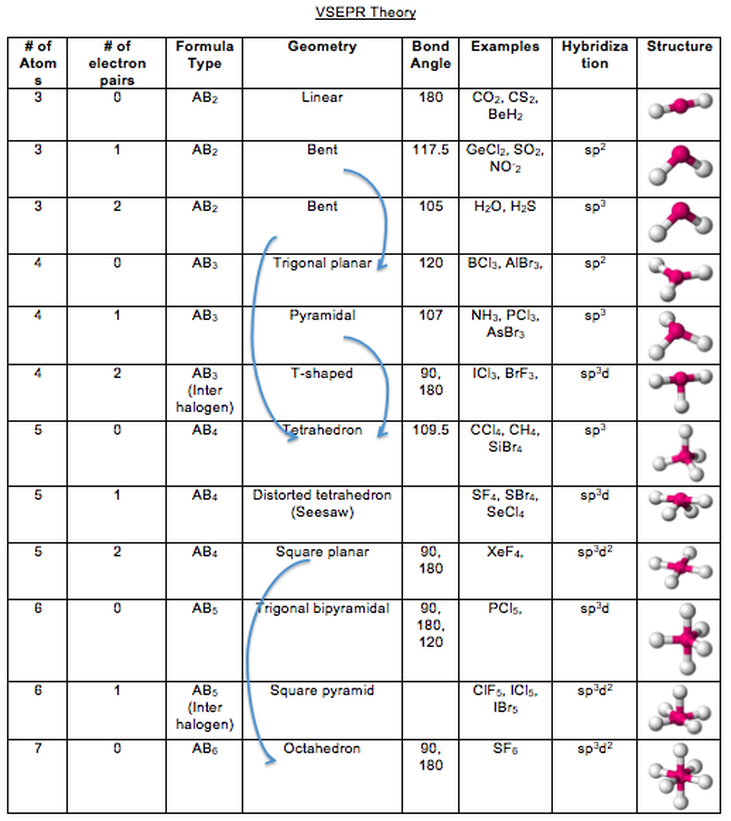
The lone pairs would be zero here and one here. The other one only has to so far to talk about lone pairs. Notice that one of these has three things. When Adam here and a lone pair there, which is also three. Let me point out how it's three for the double bond. All right? So both of these, because they have three bond sites each are gonna be SP two. Figure out what their hybridization would be. I want us to also pause the video and figure out what kind of hybridization is thes two would have. Okay, So what if I have this Adam right here in this Adam right here? Okay.
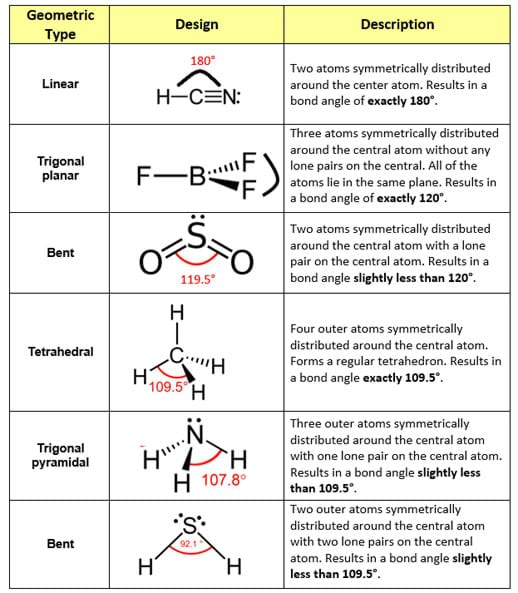
So now what I want to do is move on to other types of hybridization. Okay, So bent would be the name that I give to it when there's two lone pairs. So that's just the name of the shape, OK, And then finally what if we have to lone pairs? That means there's even less that I can see. Okay, Tribunal parameter, but most of times just called parameter. Is tribunal parameter all or what sometimes is just called parameter. Remember that electrons are tiny, so imagine that I'm looking with a microscope and I can't see the loan Parad all What is this gonna look like? Well, if you think about it, it kind of looks like a pyramid, right? Okay, think about it. Now, if I have one lone pair, what I'm going to envision is that I think for all of these names think about that. So every every bond site is an atom or is a bond. What? Least since Jenkins, the Tetra Hydro is the name given to the shape off four bond sites with zero lone pairs. Okay, so the name that I'm gonna use for zero lone pairs Does anyone know? I think I heard you say it. I want shape names that are going to relate to these. So kind of like I say that something looks like a circle or a square. Looking from three, we want words that are going to describe those shapes. Even though they're all sp three hybridized, we want words that are going to distinguish something that looks like 11 is very different. Okay? And that's where the geometry comes in. So I have one and two, and you can see that all these sp three hybrid is, but they all look different.

The second one has one lone pair, and the third one has to lone pairs. So, as you can see, this first one doesn't have any lone pairs. All right, so now what I wanna do is enter in the amount of lone pairs that each one has. Some of them have Adams and lone pairs, but that's still four bond sites total. And the reason why is because they all have four bond sites. All right, so if you were using the rules correctly, what you would have noticed is that these air all sp three, even though they look very different, they're all SP three hybridized. So go ahead and pause the video trying to figure out the hybridization for all three. And what I want you guys to do is use the hybridization summary chart and use the rules that I taught you about bond sites to figure out what kind of hybridization in these three will have. Okay, what does it look like? If I were to put it under a microscope, what kind of shape would it have? All right, so let's go ahead and start off with these first three molecules. When I talk about geometry, what I'm gonna be talking about is the actual shape of the molecule. I'm always talking about the orbital's that air hybridizing that are blending so sp two s, p three whatever. All right? And we already hinted at this when we talked about the bond angles of different atoms. And what it basically says is bond sites will repel each other as much as possible. So Vesper theory is something that you should have learned in Gen. So now let's bring this all together talking about molecular geometry and Vesper theory.


 0 kommentar(er)
0 kommentar(er)
